In the coming months the US FDA (the organisation that regulates the testing of drugs and new therapies in the USA) will decide whether to allow the large-scale experimental transplantation of pig hearts that are genetically modified to be compatible with humans.
The time seems to be coming when transplants of organs obtained from animals genetically modified for this purpose, known as xenotransplants or xenografts, will be subject - at least in the US - to the only real test that can establish how effective they are and whether or not there are serious risks: large-scale, standardised clinical trials.
Xenotransplantation: towards experimental protocols in larger numbers
To date, however, the few experiments that have been carried out have been on individual cases: in particular, on patients in dire conditions, for whom a compassionate protocol (as it is known in technical terms) has been authorised, departing from the rules that prohibit the procedure. But individual cases are not needed for approval and now doctors and researchers who have been studying xenotransplantation techniques for years argue that there are the conditions (as a critical mass of information has been accumulated) to proceed with larger numbers, within the framework of experimental protocols still to be developed.
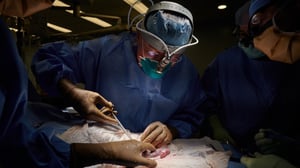
An article in the science journal Nature is dedicated to the subject. It describes the two best-known events in this delicate and controversial field: in 2021, surgeons at New York University obtained permission to transplant pig kidneys into two clinically dead patients, albeit still with beating hearts, in order to test rejection and organ function.
The kidneys worked well for 54 hours and apparently started to produce urine. More attention was then paid to the experiment conducted in Baltimore in early 2022, when a patient received a heart, also from a pig. The man, however, died two months later from a porcine cytomegalovirus infection, which was silent and therefore undetected before it was reactivated in the transplanted person.
Xenotransplantation: issues still to be solved
These two cases embody the main questions to be answered. Firstly, the source of the organs. The most suitable animal seems to be a pig, subject to appropriate genetic modifications so that it develops organs with human antigens, which will not be recognised as foreign and rejected.
All the tests carried out in recent years, even on primates other than humans (i.e. baboons) are cause for optimism, but they are not enough, because the human body’s reactions are difficult to predict. This is why there is a call for clinical studies on patients, also to optimise genetic modifications and to understand which pig breeds work best for these purposes.
Another vital aspect, as seen in Baltimore - and according to most sceptics an insurmountable one that will prevent real development of xenotransplantation - is that of possible viruses. Apart from porcine cytomegalovirus, there are probably hundreds or thousands of unknown viruses lurking in animals, even when they are reared under the best conditions, for example on specialised farms. Studies would make it possible to detect at least some of them, but it is not certain that the risk of unknown infections (with all that this entails, including possible transmission between species) can be overcome to an acceptable extent.
Finally, only clinical trials will allow researchers to develop the best cocktail of immunosuppressive (anti-rejection) drugs to be used in each patient, depending on the condition of the organ, the patient and the source animal. And it also remains to be seen whether patients’ individual diseases, like diabetes, may in turn have an influence on transplant success.
What US surgeons want
Faced with a demand for organs that has now grown to 100,000 in the United States alone (only some of which are available), American surgeons (in particular those at the University of Alabama and the University of Maryland) are currently considering extremely focused studies on specific aspects, to be carried out on people who no longer have any life expectancy.
They are discussing this with the Food and Drug Administration (the FDA, the body that regulates drug trials and other therapies in the United States). This important organisation has not yet expressed an opinion, but according to some US media, an announcement on timing and conditions is expected within a few months.
Credits:Joe Carrotta for NYU Langone Health
Surgeons at New York University transplanted pig kidneys into legally dead people on ventilators for the first time late last year.